TGA – therapeutic goods (medical device): orthosis, footwear, orthopaedic, shoe, insole
In Australia, the Therapeutic Goods Administration (TGA) regulates and oversees therapeutic goods, including medical devices like orthoses, orthopedic footwear, and shoe insoles. Here’s an overview of how these products are regulated by the TGA:
Orthoses (Orthotic Devices):
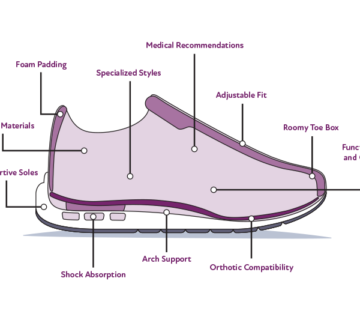
• Orthotic devices, which are also commonly referred to as orthoses or orthotic insoles, are custom-made or prefabricated devices designed to support, align, or improve the function of the feet. They are often used to treat various foot conditions or to provide support for individuals with specific biomechanical issues.
• In Australia, orthotic devices fall under the category of medical devices. Manufacturers and suppliers of orthotic devices are required to comply with the regulations set forth by the TGA.
• Depending on the type and intended use of the orthotic device, manufacturers may need to register their products with the TGA or ensure they meet certain conformity assessment requirements. The TGA provides guidance on the regulatory requirements for medical devices, including orthoses.
Orthopedic Shoes:
• Orthopedic shoes are specially designed shoes that provide enhanced support and comfort for individuals with foot deformities, structural issues, or medical conditions affecting the feet. These shoes often accommodate orthotic inserts and are tailored to the individual’s foot shape and condition.
• Orthopedic shoes are considered medical devices in Australia, and manufacturers must adhere to TGA regulations when producing and marketing them. This includes compliance with safety and quality standards.
• Manufacturers may be required to obtain TGA approval or ensure conformity with relevant standards and guidelines for orthopedic shoes.
Shoe Insoles:
• Shoe insoles, also known as shoe inserts or footbeds, are devices placed inside regular shoes to provide additional cushioning, support, or correction. They can be either over-the-counter (OTC) or custom-made.
• OTC shoe insoles are generally not classified as medical devices and may not be regulated by the TGA unless they make specific medical claims or have an intended therapeutic purpose. However, manufacturers must still ensure their products comply with consumer safety regulations.
• Custom-made shoe insoles designed for medical purposes, such as correcting foot abnormalities or addressing specific medical conditions, may be considered medical devices and subject to TGA regulations.
Manufacturers and suppliers of these products should familiarize themselves with the TGA’s regulatory requirements, including conformity assessments, labeling, and advertising rules, to ensure compliance with Australian law. It’s important to consult with the TGA or seek legal advice when dealing with the regulatory aspects of therapeutic goods in Australia to ensure full compliance and product safety.